FDA Green-Lights Clairity Breast: How an AI-Powered Mammogram Could Rewrite Breast-Cancer Prevention
A first-in-class milestone
On June 2 2025 the U.S. Food & Drug Administration granted De Novo authorization to Clairity Breast, clearing the way for the first commercial AI platform able to forecast a woman’s five-year breast-cancer risk directly from a routine 2-D screening mammogram. Because the agency used the De Novo pathway, reserved for novel devices with no predicate, any future vendor must now meet or exceed Clairity’s performance bar.
(Original press release: BCRF blog)
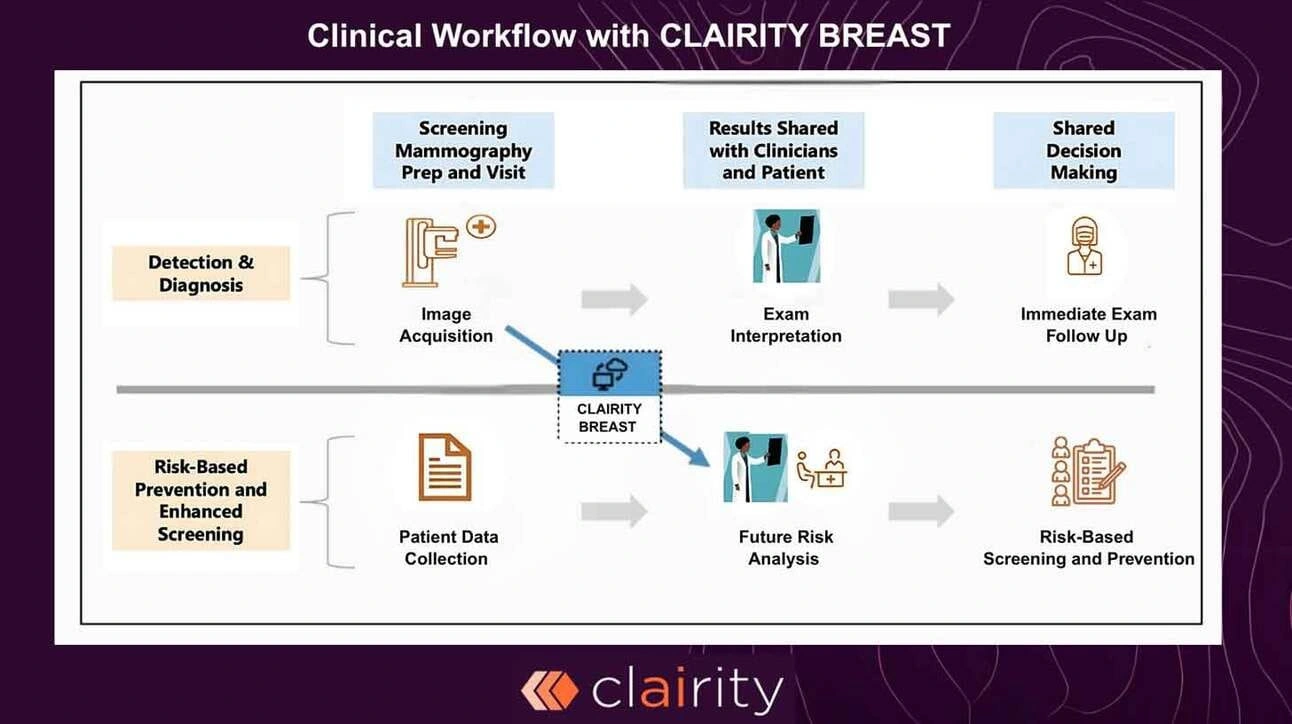
How the model works
Clairity’s convolutional neural network reads the raw mammogram, hunting for micro-patterns of tissue architecture that radiologists cannot see. It then converts those signals into an easy-to-interpret low, intermediate, or high risk score for the next five years. Key points:
- Training data: Millions of historical mammograms paired with at least five years of follow-up outcomes.
- Validation cohort: 77 000 additional exams from five geographically and demographically distinct screening sites—one of the largest prospective validations ever reported for an imaging-AI device.
- Bias mitigation: Because the algorithm sees only pixel data, it sidesteps the demographic skew that plagues questionnaire-based calculators such as Gail, Tyrer-Cuzick, or BOADICEA.
Evidence that tipped the FDA
In a pivotal reader study of 30 000 consecutive screening exams, Clairity Breast flagged:
- 37 % of women in their 40s as intermediate-risk
- 16 % of women in their 40s as high-risk
Those risk distributions mirror women a full decade older, challenging age-only screening paradigms and aligning with the U.S. Preventive Services Task Force’s recent draft guidance to begin biennial screening at 40.
Why clinicians (and payers) should care
| Current paradigm | Risk-adapted paradigm unlocked by AI |
|---|---|
| Age-based screening intervals | Personalised interval and modality |
| One-size-fits-all follow-up | MRI, ultrasound, chemoprevention or genetics only when the AI score justifies it |
| Over- and under-screening common | Potential to cut false positives in low-risk women while catching high-risk cancers sooner |
Beyond clinical upside, imaging centers could gain a new reimbursable CPT code once payers adopt risk-stratified screening. (At launch, women will pay cash while insurers evaluate coverage.)
Equity baked in
Roughly 85 % of women who develop breast cancer have no family history. Traditional risk models miss many of them—especially women of color—because those models were trained on overwhelmingly Caucasian datasets. Clairity enforces proportional representation during training and requires only the mammogram, making it deployable even in safety-net clinics lacking genetics counselors or detailed electronic records.
Roll-out timeline
- Q4 2025 – Commercial launch in U.S. imaging centers; integration is a software upgrade compatible with existing PACS/RIS.
- 2026 – Target for Medicare and major-payer coverage decisions.
- Global expansion – CE-mark application in preparation, with early partnerships forming in Australia and the U.K.
The bigger picture
Clairity Breast is more than a single product; it’s proof that AI can flip imaging from a diagnostic to a predictive discipline. Similar pixel-based risk tools are already being explored in CT lung cancer screening, coronary calcium scoring, and liver ultrasound. Regulators will likely lean on Clairity’s precedent when evaluating those devices.
What to do next
Radiology groups Plan workflow changes now: decide how elevated scores will be flagged to referring physicians and how outcomes will be tracked for payer negotiations.
Health-IT teams Budget for new compute: Clairity’s SDK supports DICOMweb and HL7 FHIR messaging but may require on-prem GPU servers or a cloud inference service.
Primary-care & OB-GYN practices Update patient education: risk-adapted screening will demand clearer conversations about supplemental MRI, chemoprevention, and lifestyle interventions.
Patients Ask the question: if you have a mammogram scheduled later this year, find out whether your imaging center offers AI-based risk scoring and what the out-of-pocket cost will be during the insurance-coverage gap.
Bottom line
Clairity Breast’s FDA nod marks the first time a screening image alone can unlock a forward-looking risk profile. It ushers in a proactive era where radiology doesn’t just find today’s cancers—it helps prevent tomorrow’s.